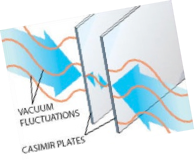
Casimir effect and van der Waals interactions. Electromagnetic filed fluctuations in confined geometries lead to an interaction between the boundaries. For ideally polarizable boundaries this is the Casimir effect. For boundaries with realistic dielectric response this si the Lifshitz- vna der Waals interaction. This interaction is essential for bio-, nano- and colloidal systems.
Research highlights

Graphics: Sci. Am., Podgornik et al. COCIS and J. Chem.Phys., Milan Hodošček and Edwards et al.Biophys. J.
Institut "Jožef Stefan", Jamova 39, 1000 Ljubljana, Slovenija, Telefon: (01) 477 39 00, Faks.: (01) 251 93 85
info@ijs.si


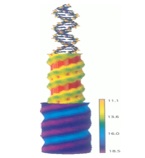
The physics of DNA. DNA is a highly charged, flexible molecule witha range of characteristic behaviors either at small (single moelcule) or large concentrations, in dense liquid crystalline  phases. This behavior in particular is quite fascinating and gives us novel insights into the packing geometry of DNA inside simple prokaryotic cell and viruses. The complicated interaction between DNA and nucleosomal proteins is the founding stone of the structure of chromatin.
phases. This behavior in particular is quite fascinating and gives us novel insights into the packing geometry of DNA inside simple prokaryotic cell and viruses. The complicated interaction between DNA and nucleosomal proteins is the founding stone of the structure of chromatin.

Interaction between lipid membranes and DNA gives rise to genosomes, DNA-lipid complexes hypothesized to play a fundamental role in gene therapy. 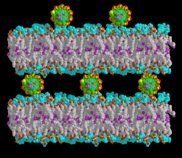 The nature of order and the interactions between both types of macromolecules are particularly important and as yet relatively poorly understood.
The nature of order and the interactions between both types of macromolecules are particularly important and as yet relatively poorly understood.


Polyelectrolytes are long charged polymers. In the presence of oppositely charged macroions they 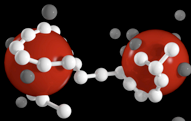 induce bridging interactions, where parts of a single polyelectrolyte chain are electrostatically adsorbed onto one macroion and parts are adsorbed onto another one. The part of the chain in between is pulling the two macroions together. Thus polyelectrolyte bridging attraction is a classical equivalent of quantum chemical bonds mediated by electrons.
induce bridging interactions, where parts of a single polyelectrolyte chain are electrostatically adsorbed onto one macroion and parts are adsorbed onto another one. The part of the chain in between is pulling the two macroions together. Thus polyelectrolyte bridging attraction is a classical equivalent of quantum chemical bonds mediated by electrons.

Polyelectrolytes in colloidal crystals sometimes behave very similarly to a gas of electrons inside a metal. In a polycation - DNA complex the density distribution of polycation  can be of two different types, depending on the charge of the polycation and the amount of Debye screening in the salt solution. Methods of solid state physics can be profitably used in order to analyze this colloid physics problem.
can be of two different types, depending on the charge of the polycation and the amount of Debye screening in the salt solution. Methods of solid state physics can be profitably used in order to analyze this colloid physics problem.