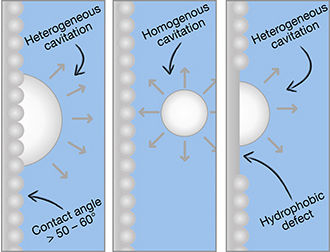
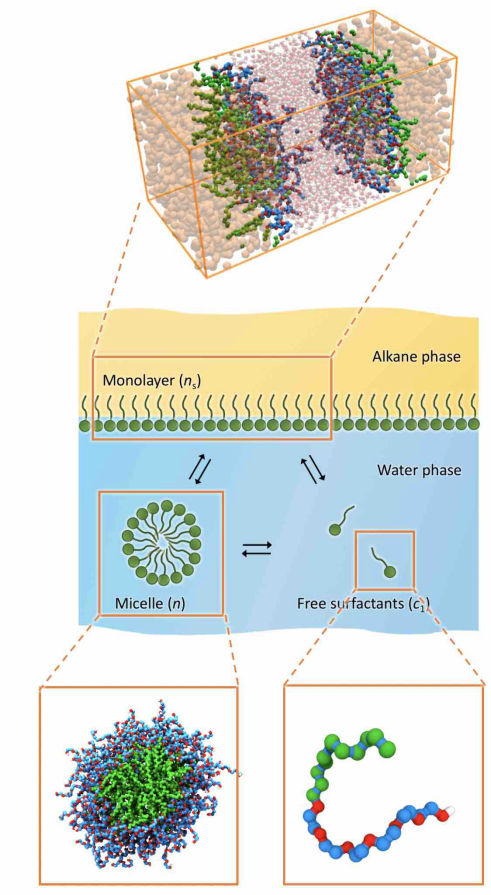
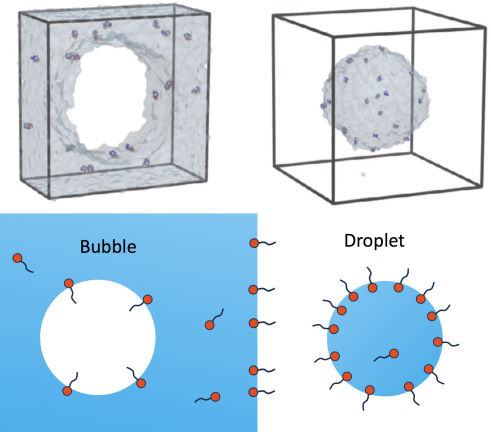
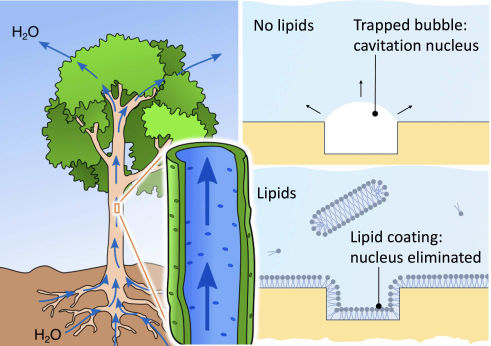
Matej Kanduč, a senior research associate at the Department of Theoretical Physics of the Jožef Stefan Institute, Ljubljana.
Research focus: Theory and molecular modeling of biological and soft-matter systems
Topics: Molecular interactions, hydrophobicity, wetting, lipids, surfactants, cavitation, simulations
E-Mail: matej.kanduc@ijs.si